MaxDividends Business Overview: One of the Largest Medical Device Companies in the World
Undervalued High Yield Dividend Stocks. Today: Deep dive into the world's leading medical technology company
MaxDividends Mission: Help & support everyone on the way to build growing passive income, retire early and live off dividends
MaxDividends Business Overview
Posts & Podcasts with deep dives into the top dividend stocks we hold. Key points, current statement, perspectives and consensus.
Intro
Health tech for a better future: From AI to connected care and beyond, their technologies are building a bridge to better health for more people.
Their Mission: To contribute to human welfare by application of biomedical engineering in the research, design, manufacture, and sale of instruments or appliances that alleviate pain, restore health, and extend life.
Learn about one of the largest medical device companies in the world, with operations in 150 countries.
Medtronic plc
“God has given us the ability to develop such devices for the good of humankind and to help restore patients’ lives. Surely that’s a worthy activity in the eyes of God”
Medtronic founder Earl Bakken
Key Dates
1949: Earl Bakken and Palmer Hermundslie form a partnership called Medtronic to service and repair medical equipment
1957: Bakken develops the world's first small, wearable, battery-operated external pacemaker; company is incorporated as Medtronic, Inc
1960: Medtronic purchases the exclusive rights to produce and market an implantable pacemaker known as the "Chardack-Greatbatch implantable pulse generator"
1969: An international division is formed to accommodate direct European sales
1976: Neurological Division is established
1977: Medtronic stock is listed on the New York Stock Exchange; company establishes its Heart Valves Division and introduces the Medtronic Hall mechanical heart valve
1981: The Spectrax SXT programmable pacemaker is introduced
1985: The Activitrax "rate-responsive" pacemaker makes its debut
1993: Medtronic introduces the PCD defibrillator
2001: Company introduces InSync, the first electrical device for treating congestive heart failure
Company History
Medtronic, Inc. is the world's leading medical technology company, controlling more than half of the $8 billion global heart-pacing market, which includes pacemakers and defibrillators.

The company's products and services also include implantable neurological pain, tremor, spasticity, and incontinence management systems; heart valves; catheters and stents for angioplasty; implantable drug administration systems; hydrocephalic shunts; autotransfusion equipment; disposable devices for handling and monitoring blood during surgery; and instruments and devices used in surgical procedures of the head and spine and by ear, nose, and throat physicians.
Headquartered in Minneapolis, Minnesota, the company conducts business in more than 150 countries.
Early History
Medtronic was born from Earl Bakken's part-time gig repairing medical equipment at Northwestern Hospital while studying electrical engineering. In 1949, he and his brother-in-law, Palmer Hermundslie, saw a business opportunity, quit their jobs, and launched Medtronic as a medical equipment repair service out of a small garage in Minneapolis.
The first year was rough—they even had a month where they made just $8. By 1950, they started selling products for companies like Sanborn and Advanced Instruments, which helped stabilize the business. More than half of their early sales came from representing these other companies in the region.
As Bakken worked closely with medical professionals, he began building custom devices for research. Over Medtronic’s first decade, he created nearly 100 custom devices, including defibrillators and other medical tools, setting the stage for Medtronic's future as a medical device manufacturer.
Advent of the Pacemaker
Medtronic's rise is closely tied to the development of pacemakers during the 1950s, a key moment in open-heart surgery history. Early external pacemakers had major issues—burning skin, being bulky, and needing to be plugged into a wall, which made power outages life-threatening.
In 1957, Dr. C. Walton Lillehei, a heart surgery pioneer at the University of Minnesota, needed a better solution and asked Earl Bakken for help. In just six weeks, Bakken delivered the world’s first wearable, battery-powered pacemaker. By the late 1950s, Medtronic's pacemaker was in use worldwide, earning the company recognition as a leader in biomedical devices.
The innovation didn’t stop there. Collaborating with Dr. Samuel Hunter and Medtronic engineer Norman Roth, the team developed the Hunter-Roth electrode, which required 70% less power. By 1960, the first implantable pacemaker in the U.S. was successfully used, and Medtronic secured the exclusive rights to produce and sell the “Chardack-Greatbatch implantable pulse generator,” further cementing its place as a front-runner in the medical device industry.
Rapid Growth in the 1960s
In 1960, Medtronic expanded internationally by partnering with Picker International, which had a network of 72 sales offices worldwide. Meanwhile, 14 Medtronic reps handled U.S. and Canadian sales. That year, Medtronic launched key products like the Telecor heart monitor and the Cardiac Sentinel.
As demand grew, the company moved to a larger facility in 1961. Sales surged from $180,000 in 1960 to over $500,000 in 1962, but expenses outpaced revenue, leading to losses. A $200,000 bond offering and a $100,000 loan in 1962 helped Medtronic turn a profit of $17,000 in the first quarter of fiscal 1962.
The 1965 introduction of Medicare boosted pacemaker sales, and new products, like the transvenous lead pacemaker, captured a large share of the market. By 1968, sales hit $12 million with profits exceeding $1 million.
Medtronic established a European Service Center in 1967, which handled 80% of overseas sales. By 1970, the company ended its contract with Picker International, taking full control of international sales, which accounted for 30% of total revenue. Medtronic expanded into 19 countries with new sales offices and manufacturing plants.
In North America, Medtronic acquired key distributors like John Hay & Co. in 1968 and A.F. Morrison in 1969, gaining full control of its distribution by 1973.
Continued Growth Through the 1970s
The 1970s were pivotal for Medtronic. By 1975, the company hit $100 million in annual sales and went public on the New York Stock Exchange in 1977. By the end of the decade, annual sales exceeded $200 million, with Medtronic commanding 35% of the global pacemaker market.
The company began diversifying into other areas, launching its Neurological Division in 1976 and the Heart Valves Division in 1977 with the Hall mechanical valve. It expanded globally, setting up HQs in Latin America and Europe, with manufacturing facilities in Puerto Rico, Canada, and France. Medtronic also made its first big acquisition, purchasing Medical Data Systems.
Although pacemakers remained the core of the business, innovations like smaller, more reliable models and a nuclear-powered pacemaker kept the company at the forefront.
Cardiovascular products contributed the bulk of sales, and vertical integration of pacemaker production was achieved through acquisitions like Micro-Rel (hybrid circuits) and Promeon (lithium batteries).
However, the decade wasn't without challenges. Inflation, stricter regulations, and a 1976 recall of the Xytron pacemaker hurt Medtronic's U.S. market share, which fell from 60% to 40%.
Leadership also shifted, with founder Earl Bakken stepping down as president in 1974 to become chairman, followed by Thomas Holloran and later Dale Olseth as CEO.
Diversification in the 1980s
In the 1980s, Medtronic focused on expanding its product line while maintaining its leadership in pacemakers. Reinvesting over 8% of annual revenue into R&D helped drive innovation, pushing annual sales to $300 million by 1981. Under CEO William R. Wallin, who took over in 1985, the company prioritized diversification.
Medtronic led the market with groundbreaking products like the Spectrax SXT, a programmable pacemaker that didn’t require surgery for reprogramming, and Activitrax, the first pacemaker to automatically adjust based on activity level, quickly capturing 20% of the market after its 1986 launch.
Acquisitions like Biotech in Italy and Vitatron in the Netherlands strengthened Medtronic's position in rate-responsive pacing.
The company also expanded into cardiac surgery and vascular therapies, acquiring Johnson & Johnson Cardiovascular and Versaflex Delivery Systems, both leaders in their fields.
Medtronic developed innovative products like the CapSure steroid leads and the SynchroMed Drug Delivery System, an implantable drug delivery system. Neurological devices like the Selectra and ComfortWave nerve stimulators further diversified the portfolio.
Despite rapid growth, Medtronic faced setbacks, including a voluntary recall of a pacemaker lead in 1983 and patent litigation with major players like Eli Lilly and Siemens. Sales stalled in 1985, marking the first year without growth.
However, by 1988, Medtronic had rebounded, reaching $755 million in sales and establishing itself as a major medical technology company beyond pacemakers.
Continuing Expansion in the Early 1990s
In 1991, William W. George took over as president and CEO, with sales surpassing $1 billion. Medtronic had six core areas of expertise: bradycardia pacing, tachyarrhythmia management, heart valves, cardiopulmonary, vascular, and neurological devices.
By 1992, international sales made up 40% of revenue, prompting expansions in Japan, China, and Eastern Europe, as well as a stronger focus on emerging markets like India, Latin America, and Africa.
The company’s diversification strategy continued with key acquisitions, including TUR (German pacemaker manufacturer), Bio-Medicus (leader in blood pumps), PS Medical (brain shunt devices), and DLP (cardiac cannulae).
These moves strengthened Medtronic's position in the medical technology space. By 1995, Medtronic held 49% of the pacemaker market, 32% of the implantable defibrillator market, and 75% of the nerve-related device market.
Medtronic ramped up product development, with R&D spending reaching 11% of revenue in 1995, up from 9.4% in 1990. Faster product cycles led to innovations like the PCD defibrillator (1993), the Jewel cardioverter-defibrillator, and new models of the SynchroMed Drug Delivery System.
This focus on cutting-edge technology helped Medtronic stay ahead in the competitive medical device industry.
Accelerated Development in the Late 1990s
In the late 1990s, Medtronic adopted an aggressive growth strategy to stay ahead in the consolidating medical technology industry. By 1996, revenue surpassed $2 billion, with acquisitions like Micro Interventional Systems (catheters for stroke treatment) and AneuRx (stented grafts).
New products like the Micro Jewel II defibrillator and Activa Tremor Control System for Parkinson’s further fueled growth.
Despite strong sales—pacemaker sales grew 9% and defibrillator sales jumped 27% in 1997—Medtronic struggled in the stent market, resulting in a business-related charge and layoffs in early 1998. Competition in defibrillators, particularly from Guidant, also impacted market share, though Medtronic still held 40-45% of the U.S. market.
In response, Medtronic made its largest acquisition to date, purchasing Physio-Control (external defibrillators). Additional acquisitions like Avecor Cardiovascular and Midas Rex LP helped the company grow 45% in 1998.
Medtronic was named one of Fortune's "100 Best Companies to Work For" and opened a new pacemaker plant in Europe.
Entering its 50th anniversary year in 1999, Medtronic launched major products like the Kappa 700 pacemaker and InSync stimulator for heart failure. The company made two massive acquisitions: Sofamor Danek ($3.6 billion), boosting its neurology division, and Arterial Vascular Engineering ($4.3 billion), giving Medtronic 40% of the global coronary stent market.
By fiscal 1999, Medtronic had seen 14 consecutive years of revenue growth, with nearly one-third of its revenue from international markets. The company also led in innovation, holding the most medical device patents globally from 1969 to 1998.
Medtronic aimed to invest $500 million in R&D in fiscal 2000 to maintain its competitive edge. Revenues for fiscal 2000 surpassed $5 billion for the first time, with continued focus on cardiac rhythm management, neurology, vascular devices, and cardiac surgery.
Reaching New Heights in the Early 2000s
In April 2001, William George retired as CEO after growing Medtronic’s revenues from $1 billion to $5.55 billion and its market value from $3.3 billion to $54 billion during his tenure.
He transformed Medtronic from a pacemaker maker into a diversified medical technology company. Arthur D. Collins, Jr. succeeded him, having played a key role in integrating late 1990s acquisitions.
Collins's first year saw Medtronic’s entry into diabetes and heart failure treatments. In 2001, the company spent $3.8 billion acquiring MiniMed, the leading U.S. maker of insulin pumps, and Medical Research Group, which developed implantable insulin pumps and glucose sensors. Medtronic also launched InSync, a groundbreaking device to treat congestive heart failure.
In 2002, Medtronic continued expanding with acquisitions like VidaMed ($328.6 million) and Spinal Dynamic Corporation ($254.3 million), bolstering its offerings in non-surgical prostate treatments and artificial spinal discs.
Key product launches included InSync ICD, a heart resynchronizer with a defibrillator, and InFuse, a bone-growth product for spinal fusion surgery. CareLink, a remote monitoring system for defibrillator patients, also debuted.
Despite success, Medtronic faced challenges. In 2002, it paid $175 million to settle stent patent lawsuits with Boston Scientific and was appealing a $271 million verdict in a separate stent case involving Johnson & Johnson. An investigation into alleged kickbacks to physicians further added uncertainty.
Medtronic had a record year in fiscal 2004, with $1.96 billion in net income and $9.09 billion in revenue (an 18% increase). However, the company recalled 1,800 defibrillators after malfunctions potentially caused four deaths.
To regain ground in its vascular business, Medtronic partnered with Abbott Laboratories to develop drug-coated stents, aiming for a U.S. launch by 2005.
Interesting Facts About Medtronic
Growth in Diabetes and AI-Driven Technologies: Medtronic's Diabetes division saw a 10.9% growth in Q4, fueled by the successful launch of their MiniMed™ 780G insulin pump system. Additionally, the company is pushing forward in the AI-driven surgery space, launching 14 new AI algorithms for laparoscopic and robotic-assisted procedures on their Touch Surgery™ platform.
Advancements in Neurosurgery and Cardiac Care: Medtronic is expanding its footprint in neurosurgery with its AiBLE™ ecosystem, which saw strong adoption, driving growth in the Cranial and Spinal Technologies (CST) division. They also submitted their Sphere-360™ PFA catheter for FDA approval, a big leap in treating atrial fibrillation, a common heart rhythm disorder.
Insulin Pump Recall Drama: In 2024, Medtronic had to issue a Class 1 recall (the most serious kind) for their MiniMed insulin pumps after over 2,000 injuries and one death were tied to hardware malfunctions. This was a major PR headache, with missing or broken parts causing insulin doses to go haywire.
Heart Device Recall: Medtronic recalled 350,000 implantable heart devices due to their failure to deliver the necessary shocks during arrhythmias. You could say these heart devices "missed a beat," causing a scramble to pull them off the market .
Kickback Controversy: Medtronic found itself in hot water when they were accused of providing "marketing services" to doctors as part of an alleged kickback scheme. The case was later dismissed, but it wasn’t the kind of headlines you want when you’re in the business of saving lives.
Medtronic Today
4P Analysis
Medtronic (MDT) excels in the medical technology space by mastering the four P’s—Product, Place, Promotion, and Price. Their innovative product lineup, global reach, strategic marketing, and competitive pricing are what keep Medtronic at the forefront of the industry.
Understanding how these elements work together is key to appreciating how Medtronic maintains its leadership and delivers cutting-edge health solutions worldwide.
Product
Medtronic plc specializes in the development, manufacture, and distribution of medical devices and technologies. A primary focus lies in sectors such as cardiovascular, diabetes management, and minimally invasive surgeries. The company’s portfolio spans a range of healthcare needs and includes devices such as:
Pacemakers
Stents
Insulin pumps
Surgical tools
Technological advancements in Medtronic’s products also reflect in the development of minimally invasive tool sets which reduce surgery time and improve recovery periods for patients.
This strategic product positioning not only enhances patient outcomes but aligns with global health care trends favoring less invasive medical interventions.
Significant contributions from Medtronic in the medical device sector include the innovation of the world’s first wearable, battery-powered cardiac pacemaker. Ongoing advancements and new product launches remain a priority to maintain competitive edge and market leadership.
Place
Medtronic plc commands a significant market presence globally, being operative in nearly 160 countries. The company strategically bases its headquarters in Dublin, Ireland, which serves as a central hub for its international operations and corporate strategies. This location supports Medtronic's involvement in global markets while providing operational advantages such as favorable corporate tax rates in Ireland.
The company’s manufacturing capabilities are extensively spread across key regions to cater to global demand efficiently. These facilities are primarily located in the United States, Europe, and Asia. Each location is strategically chosen to optimize production capabilities and logistics while adhering to the varied regulatory standards applicable in each geography.
Medtronic's market penetration strategy involves intricate distribution channels to ensure wide and effective coverage. Products are primarily sold through hospitals, clinics, and healthcare providers across the world, which are critical touchpoints for the company's extensive range of medical devices and services. This direct sales route is supplemented by robust partnerships with local and regional distributors, enhancing its reach further.
Direct Sales Force: Medtronic employs a specialized direct sales force skilled in comprehensive customer support and in-depth product knowledge.
Distribution Partnerships: To expand its market reach, particularly in regions where direct presence is less viable, Medtronic strategically allies with local distributors.
The geographical dispersion of Medtronic’s operations and collaborations not only maximizes its market coverage but also mitigates risks associated with market volatility and political changes, ensuring a steady supply chain and sales continuity across different regions.
Promotion
Medtronic plc operates a robust promotion strategy that integrates multiple channels, with an emphasis on both traditional and digital marketing tactics.
Direct Marketing and Advertising: Annually, Medtronic invests significantly in its marketing and advertising efforts.
Digital Marketing: The company's digital platforms and social media presences form a pivotal part of its marketing strategy, fostering engagement and outreach.
Trade Shows and Medical Conferences: Medtronic is a prominent participant in global medical conferences and trade shows, which effectively facilitate direct interactions with healthcare providers and industry stakeholders.
Educational Programs for Healthcare Professionals: Investment in education for healthcare professionals is a cornerstone of Medtronic's marketing approach, ensuring that the practitioners are well-versed in the benefits and usage of their products.
Partnerships with Healthcare Providers and Institutions: Medtronic partners with numerous esteemed institutions such as hospitals and research facilities to advance medical innovations and improve device implementation.
Patient Advocacy and Success Stories: Utilizing narrative-driven approaches, Medtronic shares numerous patient success stories annually. These case studies highlight the real-life impact of Medtronic’s technologies, providing authentic content that enhances consumer trust and engagement.
Price
Medtronic employs a premium pricing strategy, reflecting the advanced technology and high value of their medical devices. This approach is particularly evident in their latest financials, where the gross margins remain robust, indicating the effectiveness of their pricing strategy in relation to the quality and innovation of their offerings.
In different regions, the pricing model shows variation to adapt to the local economic conditions and healthcare systems.
The company also engages in strategic pricing practices such as offering volume discounts and contractual agreements with large healthcare institutions.
Volume discounts are negotiated based on the purchasing capacity of the institution, fostering long-term partnerships.
Contractual terms often include performance-based pricing models, aligning the costs to the outcomes, which is a cornerstone of the value-based healthcare initiatives Medtronic promotes.
Investments in value-based healthcare are a critical part of Medtronic's pricing strategy. These investments aim to justify their products' costs by demonstrating improved clinical outcomes. Such outcomes are pivotal in negotiating price points with healthcare providers and insurance bodies, reinforcing the company’s value proposition.
📝 Medtronic (MDT) Business Model
Key Partnerships
Medtronic plc’s robust approach to key partnerships plays a crucial role in sustaining its competitive advantage and innovation capabilities within the medical technology industry. These partnerships are multi-faceted, involving collaborations with various stakeholders such as healthcare providers, technology firms, academic entities, and insurance companies.
Collaborations with Healthcare Providers
Medtronic engages in extensive collaborations with healthcare providers, which include hospitals, clinics, and individual healthcare professionals. These partnerships are essential for several reasons:
Innovation and Product Development: Direct feedback from daily medical practitioners helps Medtronic in refining existing products and developing new solutions that meet actual clinical needs.
Training and Education: Medtronic provides comprehensive training programs for healthcare providers to ensure safe and effective use of their medical products, which also helps in maintaining product demand and enhancing patient outcomes.
Market Penetration: Collaborations help in integrating Medtronic’s technologies into standard healthcare practices, thus broadening their market reach and adoption.
Joint Ventures in Technology Development
Technology development partnerships and joint ventures stand as a pillar in Medtronic’s strategy for staying at the cutting-edge of medical technology. For example:
Co-Development Projects: Medtronic partners with technology companies, both large and small, to co-develop new technologies ranging from minimally invasive surgical instruments to wearable health devices, deriving mutual benefits from shared expertise and resources.
Access to Emerging Technologies: Through these ventures, Medtronic accesses innovative technologies and intellectual property that can be critical for maintaining technological leadership.
Partnerships with Academic and Research Institutions
Medtronic places a strong emphasis on its ties with leading academic and research institutions around the globe. These relationships bolster the company’s research and development initiatives:
Research Collaborations: Academic institutions offer a rich resource of research and innovation, supporting Medtronic in exploring breakthrough scientific discoveries that can translate into effective medical solutions.
Clinical Trials: Academic centers often participate in clinical trials, providing vital data that helps in the assessment and validation of Medtronic’s medical technologies in real-world scenarios.
Agreements with Insurance Companies
Partnerships with insurance providers are crucial for Medtronic, as they directly influence the adoption of Medtronic’s medical devices by making them accessible and affordable to patients:
Coverage and Reimbursement: Establishing reimbursement agreements ensures that patients can afford the costs of Medtronic’s devices, which is critical for the commercial success of new and existing technologies.
Value-based Healthcare Models: Collaborations often help in promoting value-based healthcare models, where payments are based on patient outcomes thus aligning the interests of Medtronic, insurers, and healthcare providers.
Key Activities
Medtronic plc's core operations encompass several critical activities that ensure continuous innovation, production efficiency, market presence, and legal compliance.
Research and Development of Medical Devices
The cornerstone of Medtronic’s business model is its robust research and development (R&D) framework. The company prioritizes the innovation and improvement of medical devices to address the complex challenges of healthcare. Their R&D process includes:
Development of pioneering technologies for various medical fields including cardiology, diabetes, and neurology.
Continual improvement of existing products based on feedback from healthcare professionals and patients.
Clinical trials and testing to ensure the effectiveness and safety of their innovations.
Manufacturing of Various Medical Equipment
Manufacturing is another pivotal activity within Medtronic’s business model, which involves the precise production of high-quality medical devices. This activity encompasses:
Production of a wide range of medical devices such as pacemakers, stents, insulin pumps, and surgical tools.
Maintenance of high manufacturing standards to meet global regulatory requirements and ensure patient safety.
Scaling of production capacities to meet global market demands while minimizing costs.
Marketing and Sales Operations
Medtronic’s market presence is strengthened by strategic marketing and robust sales operations, which are designed to maximize reach and influence in the global healthcare market:
Development of targeted marketing campaigns to educate healthcare providers on the benefits and applications of their products.
Collaboration with hospitals, clinics, and other medical facilities to facilitate the adoption of new technologies.
Extensive training programs for sales teams to ensure they are well-versed in the features and benefits of each product.
Regulatory Compliance Management
Compliance with regulatory standards is critical in the medical device industry. Medtronic engages in comprehensive regulatory compliance management to uphold the highest standards of safety and efficacy. Key aspects of this activity include:
Navigating the complex landscape of international medical device regulations.
Ensuring all products comply with the regulatory standards of each country where they are marketed.
Maintaining up-to-date certifications for all products and manufacturing facilities.
Each of these key activities is crucial for Medtronic to sustain and enhance its market leadership, ensuring that they continue to deliver innovative solutions that advance the efficiency and effectiveness of healthcare around the world.
Key Resources
Medtronic plc's strong positioning in the medical device industry is driven by a variety of key resources that enable the company to design, manufacture, and market its advanced medical devices. These resources are pivotal in sustaining its competitive advantage and are categorized into the following core areas:
Advanced Technology for Device Manufacturing
The cornerstone of Medtronic’s success is its commitment to innovation and state-of-the-art technology in device manufacturing. The company invests heavily in research and development to pioneer technologies that improve patient outcomes and streamline healthcare processes.
Skilled Workforce in Engineering and Healthcare
Medtronic’s ability to innovate and maintain product excellence is significantly supported by its skilled workforce. The company employs a diverse pool of professionals ranging from biomedical engineers to clinical specialists who bring a wealth of knowledge and expertise in both engineering and healthcare sectors.
Strong Intellectual Property Portfolio
Maintaining a robust intellectual property (IP) portfolio is crucial for Medtronic’s sustainable growth. The company's IP rights protect its innovations and provide a competitive edge by preventing other companies from replicating its technology.
Global Distribution and Supply Chain Network
Medtronic’s expansive global distribution and supply chain network facilitate the effective delivery of medical devices to markets worldwide. This network includes manufacturing sites, distribution centers, and logistical frameworks designed to meet the demand in various global regions efficiently.
Value Propositions
Medtronic plc's value propositions are centered around delivering high-quality, innovative medical solutions that enhance patient outcomes and ensure safety. Its business model emphasizes the development and distribution of medical devices that cater extensively to various therapeutic areas:
Innovative Medical Devices Improving Patient Outcomes: Medtronic invests significantly in research and development to innovate products that offer superior therapeutic and diagnostic capabilities. This innovation leads to improved patient outcomes by enhancing the effectiveness of medical treatments and surgeries.
Extensive Portfolio of Products Across Various Therapeutic Areas: Medtronic's diverse product portfolio addresses a wide range of healthcare needs across numerous disciplines, including cardiology, diabetes, and neurological disorders.
Reliable and High-Quality Medical Equipment: The company adheres to stringent quality control measures to ensure that each and every device is reliable and functions optimally. Medtronic's reputation for quality is not only a key component of its brand identity but also crucial for building and maintaining trust with healthcare providers and patients.
Strong Focus on Patient Safety and Compliance: Medtronic prioritizes patient safety and regulatory compliance in all its engineering and manufacturing processes. The company actively works to exceed international safety standards, reflecting its dedication to patient care.
Customer Relationships
Direct Support to Healthcare Professionals
Medtronic recognizes the critical role that healthcare professionals play in delivering effective patient care. The company establishes robust relationships with these professionals by providing ongoing support, which includes direct access to dedicated field service personnel and clinical specialists
Patient Education and Engagement Programs
Medtronic is committed to improving patient outcomes through comprehensive patient education and engagement programs. These initiatives are designed to empower patients by providing them with the knowledge and tools needed to manage their health conditions effectively. Programs typically include:
Informational brochures and online resources that cover disease states, treatment options, and device functionality.
Interactive online platforms that allow patients to track their health progress and communicate directly with healthcare providers.
Educational workshops and seminars led by healthcare professionals.
Training Programs for Device Operation and Maintenance
To ensure safe and effective operation of its medical devices, Medtronic provides extensive training programs aimed at healthcare professionals. These programs are critical for:
Enhancing the technical skills of healthcare providers in using advanced medical devices.
Ensuring devices are operated with precision to avoid any complications during procedures.
Maintaining the equipment properly to extend its lifespan and reduce operational costs.
Channels
Medtronic plc implements a multi-channel distribution strategy to enhance its market penetration and ensure the availability of its medical devices to healthcare providers worldwide. This strategic approach is designed to meet the diverse needs of its global customer base effectively.
Direct Sales Force
The backbone of Medtronic's channel strategy is its robust direct sales force. This team is strategically positioned in key markets around the world, providing specialized knowledge and support tailored to the local needs of hospitals and healthcare facilities.
Online Platforms for Product Information and Purchasing
Medtronic also leverages technology through its online platforms. These platforms serve dual purposes—providing extensive product information and enabling e-commerce capabilities. Customers can access detailed product descriptions, instructional videos, case studies, and peer-reviewed research to aid their purchasing decisions.
Distributors and Resellers in Various Countries
Recognizing the vast geographical and regulatory diversity, Medtronic partners with distributors and resellers to extend its reach. This network is especially crucial in countries where Medtronic does not have a direct presence.
Participation in Medical Conferences and Exhibitions
Lastly, Medtronic actively participates in medical conferences and exhibitions globally. These events offer a platform for direct engagement with healthcare professionals and facilitate the showcasing of new technologies and products.
Customer Segments
Medtronic plc strategically focuses on diversified customer segments that are intrinsic to the medical device industry. These segments play crucial roles in the company's business operations, influencing both the development of products and the strategies for market penetration and expansion. The primary customer segments targeted by Medtronic plc include:
Hospitals and healthcare facilities: This segment includes large hospitals, specialty clinics, and general healthcare facilities that require a continuous supply of medical devices for the treatment of various conditions. Medtronic provides a broad range of critical devices, such as cardiovascular products, neuromodulation devices, and surgical technologies, which are essential for these institutions to function efficiently and effectively.
Clinicians and medical professionals: Clinicians, including surgeons, physicians, nurses, and other healthcare providers, influence or directly decide the purchasing decisions in healthcare institutions. Medtronic focuses on equipping these professionals with advanced tools and technologies that enhance diagnostic abilities, therapeutic outcomes, and overall patient care.
Patients needing therapeutic and diagnostic devices: This segment is ultimately the end-user of Medtronic's products. By addressing patients' needs and focusing on patient outcomes, Medtronic not only builds its reputation within industry circles but also among consumers.
Health insurance providers: Insurance companies play a critical role in the healthcare ecosystem, often deciding the accessibility and affordability of various treatment options through their coverage policies. Medtronic engages with this segment to ensure that their devices are covered by insurance plans, enhancing their accessibility to patients.
By understanding and prioritizing the needs and expectations of these customer segments, Medtronic plc can more effectively tailor its product offerings, marketing strategies, and sales approaches to different market demands and dynamics.
🧮 Cost Structure
Medtronic plc's cost structure is a result of its comprehensive operational scope within the medical technology industry, encompassing research and development, manufacturing, quality control, marketing, and regulatory compliance. The following analysis breaks down these major cost components:
High Expenditure on Research and Development (R&D):
As a leader in medical technology, Medtronic prioritizes innovation to stay competitive. Investing in R&D allows the company to develop new and improved medical devices and solutions that address the complex needs of healthcare providers and patients.
Costs Related to Manufacturing and Quality Control:
Medtronic's commitment to high-quality medical devices necessitates substantial investments in manufacturing and quality control. These costs encompass the procurement of raw materials, operation of manufacturing facilities, implementation of advanced manufacturing technologies, and employment of sufficient personnel to ensure operations adhere to regulatory standards.
Marketing and Sales Expenses:
Market penetration and the successful launch of new products require extensive marketing and sales efforts. Medtronic incurs significant expenses in these areas, including advertising, promotional activities, sales force training and compensation, and participation in trade shows and medical conferences.
Regulatory Compliance and Legal Costs:
Navigating the global regulatory landscape incurs considerable costs. Medtronic must ensure compliance with diverse medical device standards and regulations across different nations. Expenses include registration, compliance testing, and certification costs.
Overall, the described components of Medtronic's cost structure are shaped by the demands and necessities of operating in a technically intricate and heavily regulated sector such as medical technology.
💵 Revenue Streams
Sales of Medical Devices and Equipment
Medtronic's primary revenue stream is derived from the sale of medical devices and equipment across multiple business segments, including Cardiac and Vascular Group, Minimally Invasive Therapies Group, Restorative Therapies Group, and Diabetes Group. Each segment offers specialized products such as cardiac monitors, insulin pumps, surgical tools, and neurostimulation devices.
Licensing of Patents and Technology
Medtronic capitalizes on its extensive portfolio of patents through licensing agreements. By allowing other companies to use its patented technologies, Medtronic generates revenue from royalty payments and licensing fees.
Service Agreements and Maintenance Contracts
Alongside physical products, Medtronic offers comprehensive service agreements and maintenance contracts. This revenue stream includes everything from device installation and calibration to repair and technical support.
Revenue from Partnerships and Collaborations
Medtronic actively engages in partnerships and collaborations with other companies, research institutions, and healthcare providers. These partnerships often lead to the development of new technologies and solutions that can be commercialized.
📊 Medtronic Financials Today
Medtronic has weathered numerous challenges over the years, including product recalls like the 2024 insulin pump incident, as well as legal disputes over faulty devices. Despite these hurdles, they’ve continued to innovate, particularly with advancements in AI-driven medical technologies.
Now that we’ve looked at their resilience, let’s dive into their financial performance over recent years to see how they’ve navigated these obstacles from a financial perspective.
Quick MaxDividends Overview
🟢 The company is currently profitable, according to the latest reports.
🟢 The company's sales are growing steadily, which contributes to its good prospects
🟢 Operating profit is growing, the company in this sense has a good margin of safety and dynamics
🟢 Earnings per share have a positive trend and are growing. This is a good sign of healthy business
🟢 The business appears to be well managed and the company has been consistently generating income and has been sustainable for many years
Key Competitors: Guidant, St. Jude Medical, Johnson & Johnson, Zimmer Holdings, Boston Scientific, Stryker, Edwards Lifesciences.
Details
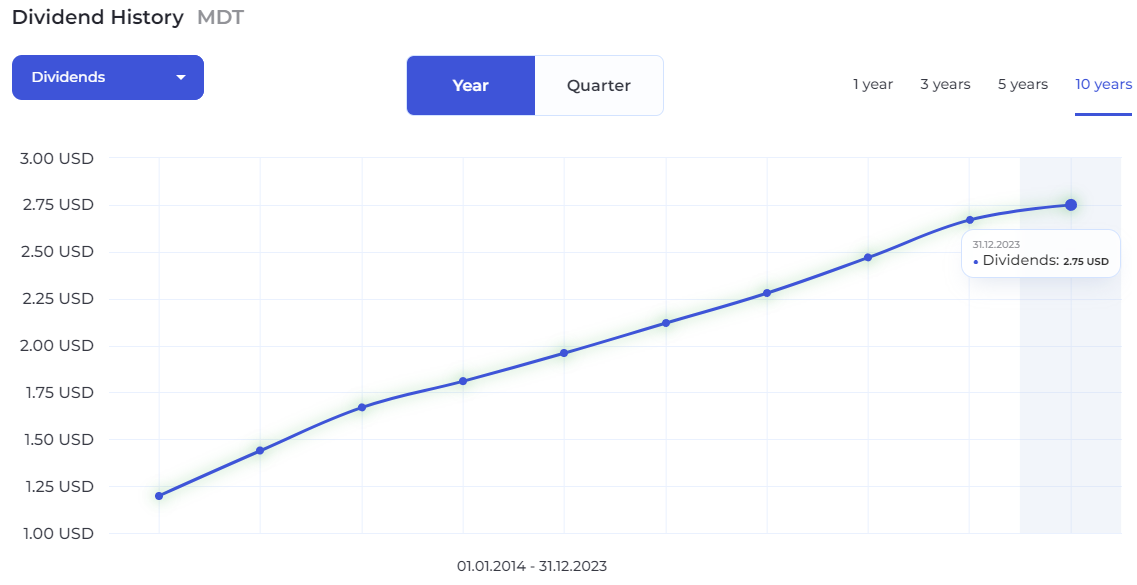
Medtronic has a history of paying dividends since 1995.
Medtronic's dividend yield of 3.15% is above the Healthcare sector, the industry and its peers average. Compared to its Healthcare sector average of 1.95%, Medtronic's dividend yield is 62% higher. The historical 5-year average of MDT's dividend yield is 2.7%, which is below the current yield.
Conclusion
An excellent company for long-term investment with strong positions and ambitious development plans.
🗓 Market expectations
As we head into late 2024, Medtronic PLC continues to deliver stable financial performance, albeit with a few areas of concern.
Earnings and Revenue Growth: In Q2 FY24, Medtronic reported revenue of $8.0 billion, marking a 5.3% year-over-year growth.
However, despite the solid top-line performance, the company's non-GAAP EPS of $1.25 slightly fell below analyst expectations, reflecting a 4% decline.
The cardiovascular and neuroscience segments were the standout performers, posting mid-single-digit growth.
Guidance and Analyst Sentiment: Looking ahead, Medtronic raised its full-year guidance for FY24 based on strong product launches and geographic growth.
Analysts remain optimistic, with many maintaining a "buy" rating and setting a 12-month price target averaging $93.4, signaling a potential upside of around 6%.
The company’s competitive edge in innovative medical technologies like the Aurora EV-ICD™ system and other new approvals bolster confidence in its long-term growth prospects.
In short, Medtronic remains a strong player in the med-tech space, with solid growth ahead despite minor earnings setbacks.
💡 Final Thoughts
Medtronic plc deftly maneuvers the complexities of the medical device market through its strategic marketing mix. Product innovation remains at the core, complemented by a robust distribution network that spans globally.
The company's focused promotional activities enhance its visibility and appeal in the competitive landscape, while strategic pricing ensures accessibility and market penetration.
Overall, Medtronic's approach exemplifies a well-balanced strategy, crucial for its enduring success and leadership in the industry.
Best regards, MaxDividends Team
More Business Overview
MaxDividends Idea
👉 My High Yield Dividend Growth story. $12,000 monthly for 120 months
MaxDividends Key Concept
Predictability in important things is the foundation of peace of mind. Peace of mind is the basis of financial well-being. MaxDividends is all about peace of mind.
At MaxDividends, we focus on a dividend growth strategy. It’s a great fit for investors who want capital appreciation, a decent level of safety, and growing income.
As the name suggests, dividend growth investing is all about finding stocks that pay dividends and can keep growing them over time.
MaxDividends Stocks pay sustainable dividends by decades without interruption, rasing them consistently over years.
MaxDividends Stocks are Unique. They not only provide income but have a strong track record of increasing that income over the years. Plus, investing in companies that consistently raise dividends has historically been a solid strategy.
Do you know what is the power of the MaxDividends Key Concept? It is that even if you stop investing right now, your dividend income will continue to grow.
This is how Max Dividends operates: No worries about price fluctuations, no concerns about stock market noise.
MaxDividends Mission
Help & support everyone on the way to build growing passive income, retire early and live off dividends.
Build your own dividend machine with MaxDividends to launch growing passive income, retire early and live off dividends with community like-minded
MaxDividends Community
MaxDividends is subscriber’s supported newsletter with community and community member’s tools to start building long-term growing passive income to live off dividends and retire early.
MaxDividends App
We’re building the best app in the world for dividend investors to help everyone on the way retire early and live off dividends.
We are recommended on Substack!
The European Value Investor ⭐️⭐️⭐️⭐️⭐️
“If you are interested in dividend strategies or passive income strategies, this Substack ia great! Its worth a visit“
Johan Lunau - The Long View ⭐️⭐️⭐️⭐️⭐️
“Practical dividend focus. Saving for retirement.“
Shailesh Kumar - The Astute Investor’s Calculus ⭐️⭐️⭐️⭐️⭐️
“Love the concept and the execution. If you are into building a portfolio that will generate ever growing income for you, subscribe to Max Dividends. I did.“
MS Cliff Notes ⭐️⭐️⭐️⭐️⭐️
“A useful substack for those that invest in stocks that pay dividends or are looking to get into such a strategy. Includes education and actionable ideas.“
Timothy Assi - Panic Drop ⭐️⭐️⭐️⭐️⭐️
“Great for helling you pick up high-yield dividend growth stocks“
And 50+ more other great authors and pro’s are recommend MaxDividends!
MaxDividends Premium: What You’ll Get
+ Bonus - access to full list of MaxDividends stocks updated weekly. Boost your passive income for living off dividends.
Unlock Top-notch dividend growth investment ideas and insights, handpicked to help you crush your financial goals, retire early and live off dividends.
NEW! List of the Most Dangerous Dividend Stocks You Should Avoid to Invest In Right Now
Stay away from dividend-trap companies with the most accurate list of dangerous stocks updated weekly
Ready-to-go step by step weekly guide to achieving financial freedom.
Grab ready-made MaxDividends stock sets starting at $300, $500, or $1000 each week.
Posts & Podcasts with deep dives into the top dividend stocks we hold. Key points, current statement, perspectives and consensus.
My personal MaxDividends portfolios and short list with all the changes and updates weekly.
Comprehensive tools to help you on the way retire early and live off dividends.
Community Like-Minded Access
Stay in touch with me and other MaxDividends followers. MaxDividends community chat of like-minded who wants to live off dividends and retire early. Discuss ideas, share insights, build plans and set goals. Support, motivation, like-minded - all in!
FAQ
👉 What is MaxDividends project idea?
👉 What is MaxDividends strategy?
👉 How do you choose MaxDividends stocks?
👉 How well does the MaxDividends strategy work for building a growing passive income?
👉 One more secret sauce of MaxDividends strategy
👉 MaxDividends Roadmap to Early Retirement with Living Off Dividends